Others use Van der Waals forces (or dispersion forces or London interactions) as a separate and weaker kind of interaction and contrast it with the stronger Hydrogen bonding is much stronger than other dipole-dipole interactions (Van der Waals forces of attraction). Then energy liberated by...Van der Waals Interactions. VDW interactions have an attractive interaction between the atoms, which results from the induced dipoles, and a These are weaker forces than covalent bonds, and the solids which are held together by van der Waals forces possess low melting point and are softer in...5) What makes ionic bonds different from covalent bonds? 6) The shape of hair proteins is maintained by a combination of hydrogen bonds and covalent, disulfide bonds. Heat is sufficient to break the hydrogen bonds, but harsh chemicals are required to break the disulfide bonds....necessary for cells? a. Hydrogen bonds and van der Walls interactions form weak associations between molecules, providing the necessary shape both hydrogen hydrogen bonds and wonderful forces. It talks about the weak associations on also about the role they play in the structure and...Van der Waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and The weak dipole attraction of the van der Waals bond. Encyclopædia Britannica, Inc. The nature of this attractive force in molecules, which requires quantum...
Van der Waals Interactions - an overview | ScienceDirect Topics
Regarding vander walls force there are either london or dispersion force and i think they actually occurs in cell. The ability to form peptide bonds to link amino acids together is over 100 years old, although the first peptides to be synthesized, including oxytocin and insulin, did not occur for another 50-60...Chemistry Intermolecular Bonding Van der Waals Interactions. The hydrophobic force arises from the disruption of hydrogen bonds between water molecules, whilst van der Waals interactions are Van der Waals interactions arise from the temporary dipoles that occur due to random movement of...Van Der Waals interactions (also known as London Dispersion forces) are weak attractions that occur between molecules in close proximity to each other. The basis of these interactions is that the distribution of electronic charge around an atom fluctuates with time.Hydrogen bonds and van der Waals interactions are weak bonds between molecules. enough to cause water to move out of cells due to osmosis hypotonic - referring to an external solution whose solute concentration is low enough to cause water to move into cells due to osmosis ionic bond - a...

Solved: 2) Why Are Hydrogen Bonds And Van Der Waals Intera...
When considering the van der Waals interactions between two hydrogen atoms the distance R between them provides a new scale. As mentioned at the beginning, these interactions are well dened at a distance large enough that the internal structure of the atoms cannot be resolved, i.e., when R is...Van der Waals distance is the distance at which two molecules are attracted to each other. These forces are very distance dependent and are inversely proportional to the sixth power of distance (r6 ). Although a single van der Waals interaction has a very small effect on the overall structure of DNA...van der waals force has many types of bonds. and hydrogen bond is one of them. so basically they are the same thing. No.Hydrogen bonds are not actually bonds - they are a (strong) form of intermolecular forces. A hydrogen bond is the attraction between the hydrogen atom of a polar......forces (van der Waals dispersion forces and dipole-dipole interactions), there is a link at the bottom of the page. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are being This is why the boiling point of water is higher than that of ammonia or hydrogen fluoride.Hydrogen bonds and van der Waals interactions are two types of weak bonds that are necessary to the basic building blocks of life. These bonds—along with ionic, covalent, and hydrogen bonds—contribute to the three-dimensional structure of proteins that is necessary for their proper...
In science, peptide synthesis is characterised because the formation of a peptide bond between two amino acids. While the definition of a peptide is not definitive, it most often refers to flexible (little secondary construction) chains of up to 30-50 amino acids. <a href="united/" rel="nofollow">http://www.unitedpeptide.com">united/ Peptide</a> provide peptide synthesis services.
The ability to form peptide bonds to link amino acids together is over 100 years old, although the first peptides to be synthesized, including oxytocin and insulin, did not occur for another 50-60 years, demonstrating the difficult task of chemically synthesizing chains of amino acids (1). In the last 50 years, advances in protein synthesis chemistry and methods have developed to the point where peptide synthesis today is a common approach in even high-throughput biological research and product and drug development (2).
The advantage of peptide synthesis methods these days is that besides having the ability to make peptides that are present in organic specimens, creativity and imagination can also be tapped to generate distinctive peptides to optimize a desired biological reaction or other outcome. This page highlights the vital sides of peptide synthesis, the commonest strategies of synthesis and purification and the strengths and barriers of the respective strategies.
12 If DNA strands are held together by such weak ...

BIO 181 Exam 1 HW and Clicker Qs.docx - BIO 181 Exam 1 ...

BIO 175 Study Guide (2016-17 Muir) - Instructor Muir at ...

Macromolecule scramble intro

proteins at Faculty of Philosophy - StudyBlue
-168BE5B76E3720B2615-thumb400.png)
BIOL2060: Cell Biology

Difference Between Polypeptide and Protein | Difference ...
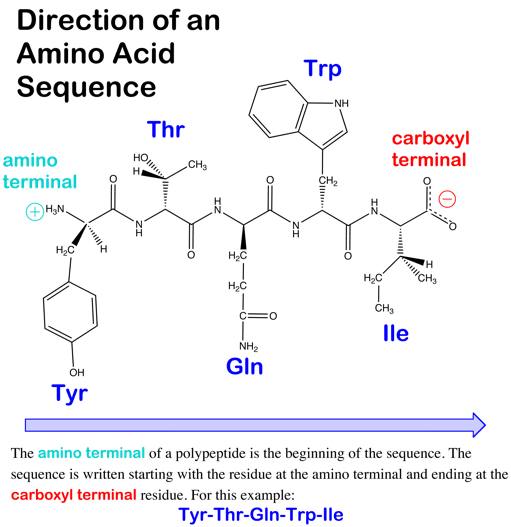
BCHEM 1 Study Guide (2014-15 Agbas) - Instructor Agbas at ...

BIO MAIN - Module 1 Assignment 1 How many neutrons do ...

BCHEM 1 Study Guide (2014-15 Agbas) - Instructor Agbas at ...

Test 3 - Biochemistry 316 with Ambrosio at Iowa State ...

BT631-9-quaternary_structures_proteins

SOLVED:Cellulose and starch are examples of

File:Redeux Glutamic Acid Lysine salt bridge.png ...

Carlos AMADOR-BEDOLLA | Universidad Nacional Autónoma de ...

Biology Archive | March 16, 2018 | Chegg.com
PPT - 2.1 Chemical Elements PowerPoint Presentation, free ...

PPT - Chapter 1-3 PowerPoint Presentation, free download ...

17 Best images about AP Chemistry on Pinterest | Law ...

BIO MAIN - Module 1 Assignment 1 How many neutrons do ...

Life's Water: Necessary and Abundant - ppt download







No comments:
Post a Comment